Phage Against the Machine
Learn more about your ad choices. Visit podcastchoices.com/adchoices
Press play and read along
Transcript
Speaker 1
Support for this show comes from Pure Leaf Iced Tea. When you find yourself in the afternoon slump, you need the right thing to make you bounce back.
You need Pure Leaf Iced Tea.
Speaker 1 It's real brewed tea made in a variety of bold flavors with just the right amount of naturally occurring caffeine.
Speaker 1 You're left feeling refreshed and revitalized, so you can be ready to take on what's next.
Speaker 1 The next time you need to hit the reset button, grab a Pure Leaf Iced Tea. Time for a tea break? Time for a Pure Leaf.
Speaker 2
Thumbtack presents project paralysis. I was cornered.
Sweat gathered above my furrowed brow and my mind was racing. I wondered who would be left standing when the droplets fell.
Me or the clawed sink.
Speaker 2 Drain cleaner and pipe snake clenched in my weary fist, I stepped toward the sink and then, wait, why am I stressing? I have thumbtack.
Speaker 2
I can easily search for a top-rated plumber in the Bay Area, read reviews, and compare prices, all on the app. Thumbtack knows homes.
Download the app today.
Speaker 3 My boss at a time, a professor called Glenn Morris, he was walking around in a very sad mood one day and I asked him what was going on.
Speaker 3 And apparently, he was treating a patient who just underwent a very sophisticated surgical procedure. I think it was organ transplantation.
Speaker 4 The surgery itself had gone well, but the patient developed an infection and the antibiotics the doctor was using just weren't working. The patient was dying.
Speaker 3 And I asked without thinking twice, you know, how come that bacteriophages didn't handle it? Because I knew bacteriophages could kill bacteria that could not be killed with antibiotics.
Speaker 3 And at that point, Glenn looked at me, you know, with that look that I realized that, you know, this is the first time he heard about this type of applications of bacteriophages.
Speaker 2 Glenn is probably not alone here. I hadn't even heard of what some people call bacteriophage or phages as they're usually called in the US.
Speaker 2 I had literally no idea what they even were until just a few years ago. It's entirely possible that this is the very first time you've ever heard of them.
Speaker 4 But we are going to be spending quite a bit of time with these tiny, potentially life-saving creatures today.
Speaker 4 Don't worry, you are in fact listening to Gastropod, the podcast that looks at food through the lens of science and history, and this story is indeed about food, I promise. I'm Cynthia Graeber.
Speaker 2 And I'm Nicola Twilley, and our phage guide this episode is Alexander Sulakulitza.
Speaker 3
It's a Georgian name. Most people call me Sandro.
Sandro is a nickname for Alexander in Georgia.
Speaker 4 Georgia is a country that used to be part of the USSR, the former Soviet Union. It's on the Black Sea and it's just north of Turkey.
Speaker 4 And phages have been used in Georgia to treat infections for almost a century.
Speaker 2 Regular people in Georgia know what phages are and that you can take them to cure infections.
Speaker 2 But in the U.S., even doctors, even the most distinguished infectious disease doctors like Sandro's boss, Glenn, they would never think to use phages on a patient with an infection.
Speaker 2 Even in a situation where antibiotics had failed and there was nothing else Glenn could use to save that person's life.
Speaker 3 And that patient died.
Speaker 3 And so it just hit me that somebody's, you know, father, brother, husband, friend just died in the most developed country in the world after undergoing some of the most sophisticated surgical procedures and died from a simple bacterial infection that could probably be treated in a developing country like Georgia.
Speaker 3 It just didn't make sense.
Speaker 4 This episode, we will start to make sense of this paradox. What are phages? What do they do? And, because we're gastropod, what do they have to do with food?
Speaker 2 We have so many questions this episode. Like, why are these phage things so popular in the former USSR, but basically unknown beyond a few researchers in the West?
Speaker 4 And why would spraying them on a hot dog make it safer to eat?
Speaker 2 This episode was made possible thanks to generous support from the Alfred P.
Speaker 2 Sloan Foundation for the public understanding of science, technology, and economics, as well as the Borrows Welcome Fund for our coverage of biomedical research.
Speaker 4 A quick note, while this episode does overall have good news and interesting science and history, as usual, it does include another death, the death of a child.
Speaker 4 The story starts at about nine and a half minutes in and goes on for about three minutes. You may want to skip that part if this topic is particularly upsetting to you.
Speaker 5
As we're sitting here talking right now, Cynthia, you have phage inside of your body. Nikki, you have phage inside of your body.
I have phage inside of my body.
Speaker 5 We all are carrying around bacteria in our microbiome, but we also have phage living there as well. Excuse me?
Speaker 4 That does sound a little alarming.
Speaker 4 It's been a while since we've spoken to him, but if you're a regular gastropod listener, you might well recognize the voice of Ben Wolf, Gastropod's in-house microbiologist.
Speaker 4 When he's not talking to us, he also heads a microbiology lab at Tufts University focused on fermented foods.
Speaker 2 And you, me, Ben, Sandro, and everybody else? Apparently, we don't just have a few phages inside our bodies.
Speaker 3 Now, humans carry billions and billions of bacteriophages. The human gastrointestinal tract, for example, of anybody,
Speaker 3 including us, carries about 10 to the 15 bacteriophages.
Speaker 4 10 to the 15th means nothing to most of us. So here it is in words.
Speaker 4 A million billion bacteriophages live in each of our gastrointestinal tracts, which also really kind of means nothing to us because this is a practically unimaginably huge number.
Speaker 3
So it's a very, very, very large numbers. And so every human being on Earth absorbs about 30 billion phages every day in our body.
So the number is mind-boggling.
Speaker 3 And just to wrap this up, using another interesting illustrative example, if we were to put all phages together, they would outweigh all elephants on Earth by a thousand-fold or more.
Speaker 3 There are more phages on Earth than every other organism, including bacteria combined.
Speaker 4 Now, you might be yelling, what the hell are these phage things that are so tiny and that we have a zillion of all around and on and in us? Good question.
Speaker 2 You might also be quietly wondering, what the hell does this have to do with food? We promise it does and in a way that could make a huge difference to us all.
Speaker 2 But first we need to figure out what phages actually are.
Speaker 5 So in a very short summary, phages are viruses of bacteria. And what they do is they attack bacteria.
Speaker 5 So just like coronavirus is a virus of humans, there are viruses that attack bacteria out in the world.
Speaker 2 When phages find a suitable bacteria, they inject their own genome inside the bacteria and take over.
Speaker 5 And that genome will be the instructions to make new phage particles.
Speaker 5 And so they'll either use some of their own machinery or even machinery of the host, the bacterium, to make more particles of themselves.
Speaker 5 And eventually, they'll burst open that host cell and all those new phage particles will be released into the environment. And then they can infect a new bacterial cell.
Speaker 4 And as the phages burst out of the bacteria, they kill it. These are teeny, tiny bacteria-killing machines.
Speaker 3 They are very mighty, but they're small and mighty.
Speaker 2
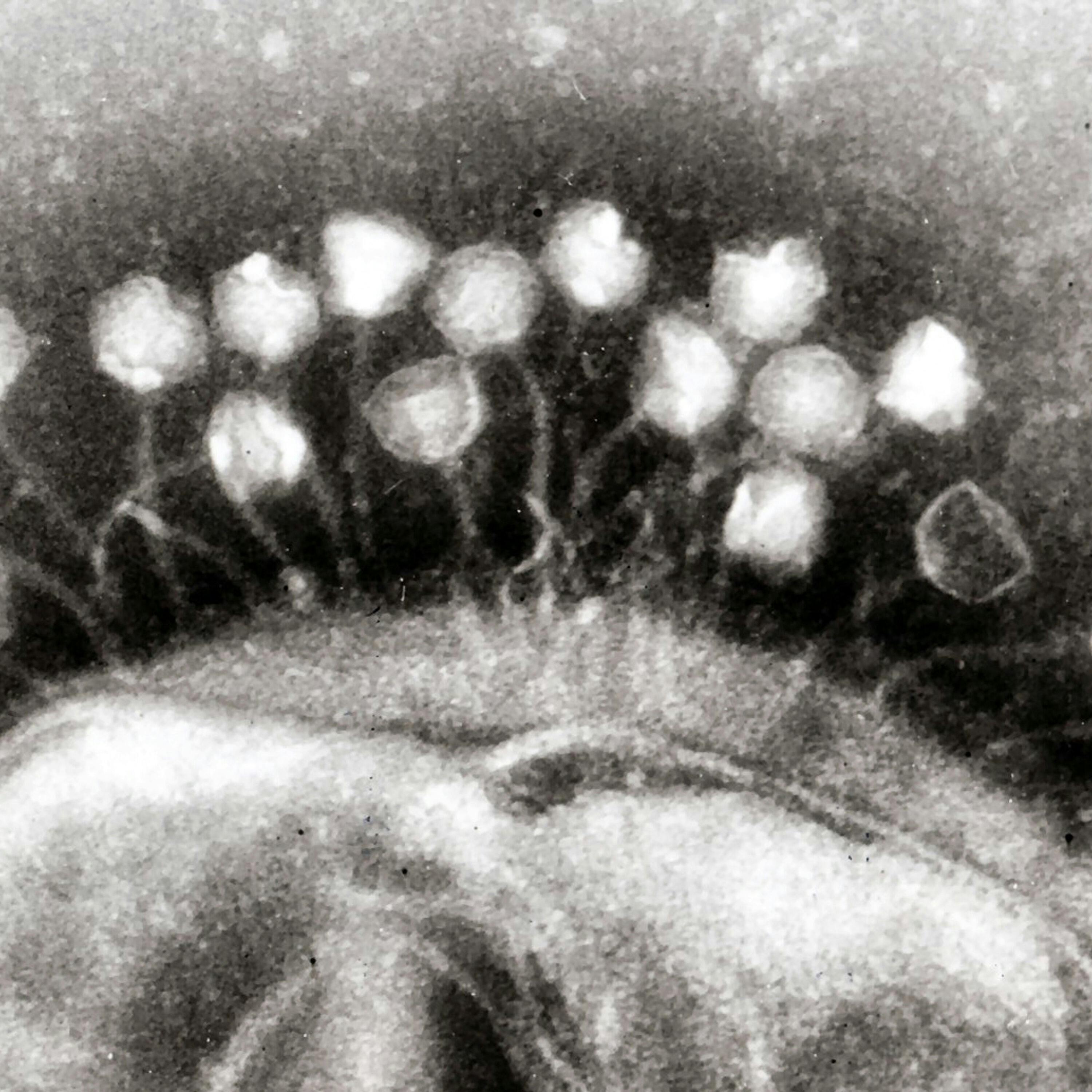
Not just small, but truly minuscule. You can't even see phages in a normal microscope.
They're a hundred times smaller than a bacteria, which is already tiny.
Speaker 4 But even though they're tiny, they're super skilled at killing bacteria, which is why they're potentially useful to us. But they were only first discovered a century ago in Paris.
Speaker 2 Back then, the technology didn't exist to allow us to see these tiny phages, but you could see what they did to bacteria. They left these giant zones of death on a Petri dish.
Speaker 2 And that was pretty exciting. Straight away, people started using phages to treat infections caused by bacteria.
Speaker 2 Elizabeth Taylor used phages for a nasty case of pneumonia she caught during the filming of Cleopatra.
Speaker 3 All that I shall ever want to hold,
Speaker 3 or look upon, or be or have is here now with you. Remember, remember.
Speaker 2 Don't want you to forget me, please.
Speaker 3 Forget?
Speaker 3 How?
Speaker 2 And she recovered sufficiently to have dozens more husbands.
Speaker 4
But then, antibiotics were discovered, and they were even easier to use than phages. So, at least in the West, antibiotics pretty much conquered phages.
Phages as a tool in medicine disappeared.
Speaker 2 But in the Soviet Union, antibiotics weren't as common, and so phages remained part of normal medical treatment.
Speaker 3 So, as I was growing up, I had taken bacteriophage myself, and almost everybody that I knew had taken them one time or another.
Speaker 3 So, I didn't think twice about them, just like you don't think twice about antibiotics.
Speaker 4 But at that moment in the early 90s in the U.S., talking to his boss and watching the patient die, Sandro realized that Americans didn't have access to this potentially life-transforming virus.
Speaker 4 Phages are everywhere in the wild, but in the U.S., they hadn't been captured and delivered as a medical tool for more than half a century. So Sandro decided to start a company.
Speaker 2 He got a team together and started to go after some funding because Sandro and his new team couldn't just import phages from Georgia. They weren't approved for use as a medicine in the U.S.
Speaker 2 No phages were. So So Sandro needed to run clinical trials and do FDA paperwork and all sorts.
Speaker 3 And we quickly found ourselves to be way ahead of time because
Speaker 3 back in those years, we're talking about late 90s, nobody in the U.S. really knew much about bacterial phagosome type of applications and it was very hard to get enthusiasts for this.
Speaker 4 So now you still might be wondering what on earth this all has to do with food. But something else happened in the U.S.
Speaker 4 in 1993, the very same year that Sandro showed up here from his native Georgia, something deadly and something tragic.
Speaker 6 It all began in January when the first of what would be more than 400 people reported that they had become sick after eating contaminated beef.
Speaker 7 It was a war zone.
Speaker 6 Seven new cases of E. coli poisoning food E.
Speaker 8 coli patients remained hospitalized at the SAR infection claimed its first life.
Speaker 2 This is a news montage from a documentary produced by Seattle's PBS station, KCTS. The New York Times also did its own mini-doc.
Speaker 6 A fast food nightmare may be getting worse.
Speaker 4 Hundreds of hospitalizations have been traced to contaminated hamburgers.
Speaker 2 Two children have died, and dozens are hospitalized.
Speaker 9 It was one of the worst food poisoning outbreaks in U.S.
Speaker 10 history.
Speaker 4 In 1993, there was an E. coli outbreak that was traced to hamburgers that were cooked at Jack in the Box restaurants.
Speaker 4 Bill Marlar was the lawyer for the families of the victims in their lawsuits against Jack in the Box.
Speaker 10 I was a young lawyer just a few years out of law school when the Jack in the Box outbreak happened, and it was pretty much the epicenter was the greater Seattle area.
Speaker 10
The outbreak outbreak was over five states, 700 people sick. There were four children who died, three of them in the Seattle area.
There were about 75 children that suffered acute kidney failure.
Speaker 10 And I wound up representing most of the really severely sick kids.
Speaker 2
E. coli is a bacteria that's really common.
It hangs out in the intestines of most warm-blooded creatures on earth and is mostly harmless. But there are some strains that can be lethal.
Speaker 2 They're called pathogenic E. coli and they can cause anything from a urinary tract infection to septic shock and death.
Speaker 4
In 1993, Darren Detweiler was a young parent and a U.S. Navy Reserve's nuclear engineer.
He had two kids, ages 9 and 16 months. The family lived about an hour and a half from Seattle.
Speaker 4 And when Darren started to see news about the outbreak, he was worried about his nine-year-old, but not the toddler, his youngest, Riley. He'd never eaten a hamburger in his life.
Speaker 11 I picked up my son from daycare.
Speaker 11 And there was a note on the door saying that the county health department found that there is a a child who was sick with E. coli, that we need to look for these symptoms.
Speaker 2 What Darren didn't realize and what most people don't know is that you don't need to eat a contaminated hamburger to contract pathogenic E. coli.
Speaker 2 All little Riley had to do was touch a surface that was contaminated with someone else's poo, someone who had the jack-in-the-box E.
Speaker 2 coli in their system, and then Riley would have put his hand in his mouth and that was enough. Riley was sick.
Speaker 11 And, you know, that night, seeing those
Speaker 11 symptoms appear, and we went to the
Speaker 11 emergency room and we were told to come back the next day. And
Speaker 11
we came back the next day. And he went from being admitted and being seen to having tests being done to a positive.
He did, in fact, have E. coli.
Speaker 11 And now he is being airlifted in a helicopter from the local hospital to Children's Hospital in Seattle. And,
Speaker 11 you know, the next day, he's he's in the pediatric intensive care unit.
Speaker 2
Darren told us that Riley had just started walking and talking. And all of a sudden, he's having huge chunks of his intestines removed.
And then the doctors are saying he's not going to make it.
Speaker 2 It all happened so fast.
Speaker 11 And that is the image that's frozen of your child for this many years.
Speaker 11 And the image of him. in my mind sitting on my lap and he was confusing an IV bag for a baby bottle and seeing him being put in a helicopter and then
Speaker 11 struggling to see him in a hospital bed when he's surrounded by machines and wires and tubes and monitors
Speaker 11 and you know we're reflecting all these years on seeing him one last time outside of the hospital in the world's smallest white coffin.
Speaker 4 That moment lives on in Bill's memory too. He represented Darren's family.
Speaker 10 One of the scenes that's sort of still seared in my memory is the Detweiler child, you know, in a little tiny coffin, you know, bearing their kid, you know, about four weeks into the Jack in the Box outbreak.
Speaker 2
This tragedy, the Jack-in-the-Box outbreak, it changed everything. Bill went from never having heard of E.
coli to being the country's number one food poisoning lawyer.
Speaker 4 And Darren changed direction entirely, too.
Speaker 11 You know, that really shaped the rest of my life in terms of
Speaker 11 I will never
Speaker 11 get my son back,
Speaker 11 but I can continue to be a father to my son in terms of the idea of making sure that that very small dash between the year he was born and the year that he died has an incredible legacy.
Speaker 11 This idea that perhaps better understanding, better meaning, better policies, better science,
Speaker 11 a better food supply system could have come out of this.
Speaker 2 Darren went back to school. He got a PhD in food regulation and compliance, and he advises the government on food safety.
Speaker 2 He's He's written books and teaches classes on how to make the food supply safer so that 16-month-olds aren't killed by burgers they've never even eaten.
Speaker 4 That's been Darren's goal for the past two decades, but in the two decades that Bill has been representing families of victims, well, that better world isn't here yet.
Speaker 10 You know, unfortunately, I always thought that, you know, the jack-in-the-box case was going to be the worst case I ever dealt with from a tragedy point of view.
Speaker 10 And, you know, unfortunately, that's not been the case.
Speaker 10 I have had cases where children have died or have become horribly injured for the rest of their lives, requiring kidney transplants or brain injuries.
Speaker 10 I'm representing a child now that the illness happened in late 2018.
Speaker 10 He's now four, but he can't walk, can't talk, can't feed himself.
Speaker 2
This is shocking. Food poisoning? I've always kind of thought of it as an upset stomach, some diarrhea.
It's gross, but whatever. But it's actually a huge issue.
Speaker 4 The official numbers are probably an underestimate because most people don't report when they have food poisoning.
Speaker 4 But the general thought is that there are about 48 million illnesses a year due to foodborne bacteria, 130,000 hospitalizations, and 3,000 deaths.
Speaker 4 And the cost to businesses and to society is somewhere between $1.5 and $7 billion.
Speaker 2 So, what is going on here? Why do we have such a huge food poisoning problem? And what do phages have to do with it?
Speaker 2 With the Spark Cash Plus card from Capital One, you can earn a limited 2% cash back on every purchase. And you get big purchasing power so your business can spend more and earn more.
Speaker 2 Stephen Brandon and Bruno, the business owners of SandCloud, reinvested their 2% cash back to help build their retail presence. Now, that's serious business.
Speaker 2 What could the Spark Cash Plus card from Capital One do for your business?
Speaker 4 Capital One, what's in your wallet?
Speaker 2 Find out more at capital1.com slash spark cash plus. Terms apply.
Speaker 2
You're basking on a beach in the Bahamas. Now you're journeying through the jade forests of Japan.
Now you're there for your alma mater's epic win.
Speaker 2 And now you're awake.
Speaker 3 Womp, womp.
Speaker 2 Which means it was all a dream. But with millions of incredible deals on Priceline, those travel dreams can be a reality.
Speaker 2
Download the Priceline app today and you can save up to 60% off hotels and up to 50% off flights. So don't just dream about that trip.
Book it with Priceline.
Speaker 4 So we first started seeing these food poisoning outbreaks in the 50s and 60s. It's not that they had never happened in the past.
Speaker 4 Part of the reason we noticed them then is we just had a much better understanding of microbiology.
Speaker 2 But also, Bill says it's quite likely that some of the worst food poisoning bacteria, bacteria like the pathogenic E.
Speaker 2 coli that caused the Jack in the Box outbreak, they didn't even exist, at least in large numbers, before the middle of the 20th century.
Speaker 4 The reason these pathogens started to emerge is that we started to raise animals in much larger groups together in a relatively small area.
Speaker 4 And that also meant manure lagoons with massive amounts of bacteria from feces all mixed up together.
Speaker 10 And it was a perfect place for pathogens to grow. And it still is a perfect place for pathogens to grow in pig farms, cattle farms, chicken farms, all of those things.
Speaker 10 those animals in close proximity are a perfect place for these pathogens to, you know, morph and breed and become more dangerous to us.
Speaker 2 So those huge animal feeding operations are encouraging the evolution of deadly bacteria, and then they're also making the resulting outbreaks much, much larger because now you have this kind of industrialized, centralized food system where contaminated burgers can get shipped around the country and cause huge national outbreaks.
Speaker 4 After the Jack-in-the-Box tragedy, E. coli was known as a hamburger disease, but it spread and now it's also a lettuce and greens disease.
Speaker 4 That's because the water and manure from these large feedlots gets onto huge farms of leafy greens. Sometimes it's used as fertilizer.
Speaker 4 Sometimes it's just because those lagoons are nearby and the contamination spreads.
Speaker 2
Divya Gironi studies E. coli at Oklahoma State University.
And increasingly, she says pathogenic E. coli is everywhere.
Speaker 12 So I would say that E. coli is not just a problem in the beef supply, but also in fresh produce.
Speaker 12 We found it in wheat flour.
Speaker 10 If you would have told me 20 years ago that romaine lettuce would be the next source of outbreaks and then unfortunately in business for Marler Clark, I would have said, you're crazy.
Speaker 10 That doesn't make any sense. But now, if you look at the pattern of outbreaks that have happened over the last 15 years, you can sort of see why it's happening because E.
Speaker 10 coli has become an environmental pathogen.
Speaker 4 You might be wondering why this problem hasn't been solved by food safety organizations. We have these organizations in the government.
Speaker 4 There's the Department of Agriculture and the Food and Drug Administration, and they're supposed to keep our food supply safe.
Speaker 2 And they have certainly tried. The Jack-in-the-Box outbreak was a giant kick in the pants.
Speaker 12 And I know that from that day onwards, I think people started realizing how big of a problem it could be. It bankrupted the company.
Speaker 12 So after that, I think USDA moved on to make it zero tolerance microorganism in the food supply.
Speaker 4
Zero tolerance means that if a food safety inspector finds even one cell of pathogenic E. coli, the entire shipment or production run is considered contaminated.
This was a big shift after 1993.
Speaker 2
Food producers and processors do have some ways to get these E. coli numbers down to zero.
For meat, they can cook the burger to a high enough temperature for long enough to kill the E. coli.
Speaker 2
For lettuce and chicken, they can wash the leaves or the carcass in chlorine, basically bleach. They can also irradiate food.
They have a few different techniques to kill these pathogenic bacteria.
Speaker 4 But these techniques can change the texture and taste of food, too.
Speaker 4 The temperature that you have to cook a burger to to kill everything off is, frankly, a little more well done than I like to eat my meat.
Speaker 4 Irradiation can have some impact on color and taste of some foods. Also, not everyone is a fan of buying food that's been washed with bleach.
Speaker 4 These processes also kill any good bacteria that might be living on our food.
Speaker 2 And meanwhile, food producers don't always follow the guidelines and use these tools properly. Killing bad bacteria adds time and expense.
Speaker 2 And the agencies tasked with enforcing these regulations, they don't have the budget to actually have enough inspectors out checking.
Speaker 4
And so food poisoning continues to be a problem today. I remember one E.
coli outbreak from Thanksgiving just a few years ago in 2018.
Speaker 13 With just one day to go before Thanksgiving, a warning, your romaine lettuce could be deadly.
Speaker 13 The CDC urging consumers to throw away any romaine grown in California's Salinas Valley, including package mixes. Then sanitize the refrigerator.
Speaker 2
And it's not just E. coli you have to worry about these days.
There's salmonella in your eggs and your peanut butter.
Speaker 3 Peanut butter.
Speaker 14
It's a multi-billion dollar a year industry and one of the most popular items on store shelves. But in January 2009, it's deadly.
Peanut products are laced with the salmonella bacteria.
Speaker 14 It's sickening and even killing people from coast to coast.
Speaker 14 There's a race against time to trace the source and stop what's turning into a nationwide panic.
Speaker 2 And there's listeria in your cantaloupe.
Speaker 15 The new warning from the country's leading health experts. It is about that cantaloupe and the outbreak of listeria, officially the deadliest food crisis in America in more than 10 years.
Speaker 4 The numbers of cases just aren't going down.
Speaker 3 We still have 48 million cases a year, every year.
Speaker 3 So there are additional approaches needed.
Speaker 2 Sandro, if you remember, started out trying to get a company going to tackle antibiotic-resistant bacteria with phages. And he was getting absolutely nowhere.
Speaker 3 And so at that point, the founders of the company sat around the table, probably drunk some whiskey from what I vaguely remember, and said, you know, how do we move this forward?
Speaker 3 Because this is clearly technology that has a place and needs to be here.
Speaker 3 And I think that the philosophical decision we made that we thought it's too early for us to push for clinical applications, we should switch to agricultural applications.
Speaker 3 And that's exactly what we did.
Speaker 4
And this turned out to be a a smart move for Sandro. We've explained that phages target bacteria cells, not human cells.
They can't hurt or damage human cells at all.
Speaker 4 But one thing you need to know about why phages might be great to fight food poisoning is that each particular phage species targets just one particular bacterium species, like a lock and key.
Speaker 2 Bacteria are really different from one another, and one of the main ways they're different is their cell wall or skin.
Speaker 2 Different species of bacteria have a completely different outer wall that separates them from the world, and so different species of phages have evolved to be able to lock on to those different skin types and penetrate them.
Speaker 3 To put it in a perspective, bacteriophage that kills, for example, E. coli, a household name, will not kill another bacteria, say Listeria monocytogenes, and vice versa.
Speaker 2 And actually Listeria was the first target that Sandro and his new company called Introlytics picked.
Speaker 3
There are a number of reasons why we picked it. It doesn't trigger most of foodborne illnesses.
In fact, it's relatively rare.
Speaker 3
But it had one of the highest, if not the highest, fatality rate or mortality rate. So just in a sensitive population, it could be as high as 20 to 30 percent.
That's almost like black plague.
Speaker 3 And so it's very, very dangerous for certain populations, like pregnant women, for example, elderly, immunocompromised elderly. So they're at high, very, very high risk.
Speaker 4 Sandra went searching for the listeria-killing phages where he'd be likely to find them. In water contaminated with a lot of feces.
Speaker 4 That's where the bacteria would be and so that's where their killers would live too.
Speaker 3 At that point when we formed the company we were located in downtown Baltimore and so most of the our initial bacteriophages came from waters of inner harbor or chesapeake bay so we would just go out get some water and find bacteriophages in that water.
Speaker 3 Then of course you find many of them and then the tricky part is to select the good one.
Speaker 2 Tricky but not as tricky as getting FDA approval for this new food safety technology.
Speaker 4
Sandra did actually find the right phage to kill Listeria but the FDA had a lot of questions. They had no experience with phages.
They didn't even know that much about them.
Speaker 4 So it took the feds four years to approve it.
Speaker 3 I think in somewhere in a food safety textbook, it has its small place in history because it was the first ever bacteriophage product that was approved by the FDA ever for food safety applications.
Speaker 2 The name of Sandro's first ever phage food safety product is List Shield, and it hit the market in 2006 with some extremely nerdy advertising.
Speaker 16
Bacteriophages are completely safe for humans and have no effect on the taste, texture, or aroma of the food. Bacteriophages.
Making food safer, nature's way.
Speaker 4 Fantastic, but I still have a lot of questions. How do you grow phages? What's the best moment in the food production process to use them? How do you even get them on the food?
Speaker 2 It's all surprisingly kind of straightforward. Sandro says they just grow the phages in biosecure tanks filled with pathogenic listeria.
Speaker 2 The phages lock onto their target, insert their DNA and multiply again and again.
Speaker 2 And then Sandro and his team centrifuge and purify the liquid so there's only phages, no nasty listeria or other microbial bits and pieces. And then they just keep the phage in water as a liquid.
Speaker 4 And then that water is sprayed directly onto the food to kill any nasty bugs.
Speaker 3 The typical application is the spraying. Now, some companies do a little bit of a dipping as well, but majority is the spraying.
Speaker 4 But I'm still wondering when in the process would be the best time for spraying, like when a plant is in the field or when meat is being packaged.
Speaker 2 Yes, in fact, Sandra says you can use phages at all stages of the process. You can spray your cheese factory with phages and get rid of any bad bacteria in the factory environment.
Speaker 2 You can give cows phages before they're slaughtered to wipe out any bad bacteria they might be carrying.
Speaker 4 But Sandra says at the moment the most effective use of phages is to spray them right at the point that food is being prepared for shipping after it's been sliced sliced and before it's packaged.
Speaker 3 There are many reasons why we do this. One of the reasons is that we would like them to be as close to the final so there is reduced risk of recontamination before we apply bacteriophage.
Speaker 2 Sandra's phages are now being sprayed on salami and hot dogs and chicken breasts and melon cubes right before the package is sealed all over the United States.
Speaker 2 And based on his research, which the FDA accepted as part of the approval process, his phages are significantly reducing listeria levels on all those foods.
Speaker 2 He says he sees between a hundred to a thousand-fold reduction in listeria levels on foods that have been treated with his phages.
Speaker 4 And how much of an impact is this having on our health? So, the Department of Agriculture modeled how many lives could be saved with a ten-fold reduction in listeria.
Speaker 4 That's much less than the effectiveness of Sandro's phages.
Speaker 3 They showed that if we were able to reduce listeria monocytogenesis levels in certain ready-to-eat meats or foods by ten-fold,
Speaker 3 they would reduce mortality in high-risk elderly population by 50%.
Speaker 4 So, yeah, he really is saving lives, potentially a lot of lives. And on top of that, there are a lot of ways that phages are better than the alternatives to kill harmful bacteria.
Speaker 4 Ben Wolf told us he's pretty psyched about phages becoming a tool to help keep our food safe.
Speaker 5 And the reason that it's a great idea, and I think it's super exciting, is one, is a way to replace other harsher antimicrobial compounds that are used to control pathogens in food systems.
Speaker 2 Plus, Plus, remember, phages are super specific. They will only kill their target.
Speaker 5 So the nice thing is if you're using these phages on something like a beautiful artisan cheese where you have lots of microbes and you don't want to wipe out all the bacteria, this thing would be very specific.
Speaker 5 It's a very great tool to just wipe out the thing you don't want and save all the good bacteria.
Speaker 4 And unlike overcooking or some of these other treatments, phages leave the taste and texture of our food alone.
Speaker 5 So you don't want to be putting on some thing to wipe out bacteria that makes it smell funny or taste funny or have some weird sticky appearance.
Speaker 5 And there have been quite a few studies showing that these phages, when used properly, don't change the flavor of your favorite hot dog or won't make your Camembert cheese taste strange.
Speaker 5
It's relatively inexpensive. That's another one of the pros I forgot to mention.
It's pretty easy to grow these things up. So the overall cost for producers and foods, it's not that much.
Speaker 5 So yeah, I think... I think it could be really widespread.
Speaker 2 When Ben asks around, he's found a lot of the cheese makers he works with are already using phages.
Speaker 2 Sandro's developed a whole range of phage products now, targeting other bad bacteria like pathogenic salmonella and E. coli.
Speaker 2 And he even has some competitors these days, other companies that are selling phage treatments commercially.
Speaker 4
One of those new startups comes from research that Divya did at her lab in Oklahoma. She's an E.
coli expert and she's tackling a different problem. E.
coli bacteria can get deep into the cells.
Speaker 4 cells themselves of leafy green vegetables.
Speaker 12 And that's what happened with the spinach outbreak was it didn't matter how much people washed it. It had already gone inside the leaf tissue.
Speaker 12
So that's the problem with a lot of these pathogenic microorganisms, especially E. coli.
It's very good in attaching. I mean, that's what it does in our intestines too.
Speaker 12 It quickly attaches to your intestinal cells, so it's hard to remove them.
Speaker 2 So say you have lovely organic spinach growing in soil that's contaminated with this pathogenic E. coli because it's been fertilized with contaminated manure rather than chemicals.
Speaker 2 Well, now it's basically impossible to just wash that E.
Speaker 12 coli off. Once you get to the processing, I really don't know if it's internalized, whether you can do much about it.
Speaker 2 You could still kill the E. coli by cooking it, but you don't usually cook romaine lettuce or baby spinach for a salad.
Speaker 4 Tivia wondered whether phages would be able to worm their way into all the crevices of the plant leaves and then wriggle into the cells to find their bacteria targets. She decided to find out.
Speaker 2 She took a bunch of leafy greens, some more crinkly like romaine, some smoother like baby spinach, and she dosed them with a ton of pathogenic E. coli.
Speaker 4 She tested the normal ways people try to kill E. coli like chlorine and peroxide and other antimicrobials and she sprayed some of the greens with phages.
Speaker 12 And then let that sit because, you know, you have to give phages some time to sort of attack the bacteria and go on about their business.
Speaker 2
And then, after a little bit, she looked to see how much E. coli was left, even deep inside the leaves.
The normal washes got some of the E.
Speaker 2 coli on the surface, but the results from the phage spray really blew Divia away.
Speaker 12
We saw a big difference between the spinach that was not treated with phage versus spinach that was treated with phage. So we definitely saw a huge reduction of E.
coli inside. We have even...
Speaker 12 published some pictures of scanning electron microscope where we do not see any E.
Speaker 2 coli coli inside the cell where bacteriophage has been sprayed versus the spinach that was not treated with bacteriophage divia told us that in theory we could eat a handful of those really contaminated greens from her experiment after she sprayed them with phages rather you than me to be honest but still divy is super excited about this she's already planning on commercializing her phage spray for leafy greens.
Speaker 4 So awesome. With so many phage products coming on the market and how incredibly effective they seem to be, does that mean that foodborne illness and diseases like the E.
Speaker 4 coli that killed Darren Detweiler's son and paralyzed Bill Marlar's client, are those all a thing of the past?
Speaker 9
This message is brought to you by AppleCard. Each Apple product, like the iPhone, is thoughtfully designed by skilled designers.
The titanium Apple Card is no different.
Speaker 9 It's laser-etched, has no numbers, and it earns you daily cash on everything you buy, including 3% back on everything at Apple. Apply for Apple Card on your iPhone in minutes.
Speaker 9 Subject to credit approval, AppleCard is issued by Goldman Sachs Bank USA, Salt Lake City Branch. Terms and more at AppleCard.com
Speaker 1 Support for this show comes from Pure Leaf Iced Tea. You know that point in the afternoon when you just hit a wall?
Speaker 1 You don't have time for self-care rituals or getting some fresh air, so maybe you grab a beverage to bring you back. But somehow it doesn't do the trick, or it leaves you feeling even worse.
Speaker 1 What you need is a quality break, a tea break.
Speaker 1 And you can do that with pure leaf iced tea, real brewed tea made in a variety of bold and refreshing flavors with just the right amount of naturally occurring caffeine.
Speaker 1 With a pure leaf iced tea in hand, you'll be left feeling refreshed and revitalized with a new motivation to take on what's next.
Speaker 1 The next time you need to hit the reset button, grab a pure leaf iced tea.
Speaker 2 Time for a tea break?
Speaker 1 Time for a pure leaf.
Speaker 3 You know, there are many good things about bacteriophages. They are natural, they are not genetically modified, they are environmentally friendly, they only kill
Speaker 3
the bad bacteria and not kill the others. So, and the list goes on and on and on.
But again, there are some weaknesses there, too.
Speaker 2 Sandro is maybe not your typical entrepreneur. He was very upfront about phage's problems as well as their potential.
Speaker 3 The major weakness is their specificity.
Speaker 3 So, for example, if the foods are contaminated with two pathogenic foodborne bacteria, Listeria SA and Salmonella, and I treat it with my Listeria phos, I may take care of Listeria problems, so you won't get sick from Listeria, but Salmonella is still there.
Speaker 4 Sandra says there are ways around that by figuring out what problems are the most common for which foods or by combining treatments. But another big problem is consumer perception.
Speaker 4 It just might feel a little strange to people that viruses are deliberately being added to their food, especially if they don't really understand how the viruses work.
Speaker 2 Right now, that's not a huge issue because phages don't have to be listed on the label the same way a chlorine wash isn't listed on the label. It's a cleaning tool, not an ingredient.
Speaker 5 You never see like, this has had viruses added to it when you pick up your package of hot dogs, right? There's never any indication of that because it is a processing age.
Speaker 4 But as it's being used more, people will become and are already becoming more aware of phages, and they might not be into having viruses on their food the same way that people in the UK don't want to buy American chicken because it's been washed with chlorine.
Speaker 2 The UK has voted to leave the European Union.
Speaker 17 Since the referendum, Britain's been hit with fears over Brexit. And then, there's this.
Speaker 3 Join me at Donald Trump's House of Wings.
Speaker 17 Chlorinated chicken, which, as unpalatable as it sounds, could form part of a new trade deal between Britain and America post-Brexit.
Speaker 4 I frankly never knew that American chicken was processed with chlorine, but everyone knows this in the UK.
Speaker 2 Because it became a huge deal during the Brexit negotiations, the EU doesn't allow American chicken to be imported because it's being dunked in chlorine.
Speaker 2 But now that the UK has shot itself in both feet by leaving, British consumers are scared that they're going to start being sold American chlorinated chicken, and they don't want that because who wants chicken so dirty it has to be bleached?
Speaker 4 What's interesting to me is that in the UK and even in the EU entirely, officials have found that there really isn't any health safety issue with eating chlorinated chicken.
Speaker 4
It does mostly kill bad bacteria, and there's only a small trace of the chemical left on the meat. They're more concerned that it hides bad farming practices.
Still, this did cause an uproar.
Speaker 2 And Sandra found that, at least early on, food companies were nervous that Americans might have that same negative reaction if they suddenly discovered their food was being sprayed with viruses.
Speaker 3 It's a tricky part. I think that was one of the main reasons that the industry has not really embraced it as fully as they should or could.
Speaker 3 The interest is growing, and our sales are going up.
Speaker 3 So, the clearly indication is that it's working, and people are beginning to get used used to the idea and more and more increasingly getting into this.
Speaker 3 But there are some very big player big companies that are very cautious because of the potential consumer pushback. And so that's been one of the major problems with implementing this technology.
Speaker 3 We're getting there, but it slows down the process significantly.
Speaker 2 None of these problems so far are total deal breakers, but there is a bigger issue.
Speaker 5 So just like antibiotics have led to the development of antibiotic resistance, bacteria become resistant to phages.
Speaker 12 Bacteria are extremely smart
Speaker 12 organisms, and
Speaker 12 I really have great respect for them. They will change, they will mutate.
Speaker 3 Because bacteria always find a way to survive and evolve. And so it's an evolutionary war, if you will, that they're fighting each other.
Speaker 4 Ben says there's already some evidence of this happening with Listeria.
Speaker 5 So for example, there's a study in Austrian dairies where they were studying isolates of listeria in a bunch of different dairies across Austria.
Speaker 5 And they noticed that in a couple of cases, the only time that you started seeing phage-resistant listeria was after these phage products were being applied.
Speaker 2 Sandro says he hasn't seen any evidence of resistance with his customers so far, but he's pretty sure that one day he will.
Speaker 3 And so we are taking some steps to address it ahead of time. So one approach to reduce the risk is to use phased cocktails.
Speaker 4 Cocktails sound like a great idea. I'd really like one right about now, but I don't think that's the kind of cocktail he's talking about.
Speaker 3 All our products are cocktails of bacteriophages, anywhere from three to six or eight bacteriophages in them.
Speaker 3 And we have sophisticated algorithms and programs that we developed here that allows us to select bacteriophages that have some overlapping kill activity and perhaps target different receptors on bacterial membrane.
Speaker 2 Remember that different bacteria have different skins and so different phages have evolved all kinds of different methods to lock onto and penetrate that skin.
Speaker 2 In his phage cocktails, Sandro always makes sure to include phages that have different attack strategies.
Speaker 3 So what that means that let's say bacteria develop a resistance in their receptor and one of the phages is no longer able to attach to it and therefore cannot kill it anymore.
Speaker 3 there is another phage in a cocktail or maybe two phages in a cocktail that can attach to a different receptor and still kill it.
Speaker 2 Kind of like with antibiotics, if you're just a little smart about how you use phages, resistance shouldn't actually be a huge problem.
Speaker 2 Of course, that relies on everyone being smart, not just Sandro, but it should be a solvable problem.
Speaker 4 But not all foods give phages the perfect environment they need to flourish.
Speaker 3
The more moisture, the better. The moist foods are well suited for phage applications.
You know, the drier the food gets, it gets a little more challenging. Because think about this.
Speaker 3
In order for phage to kill bacteria, it needs to attach to that bacteria. It needs to find it.
And so with moist foods, you have water that allows phages to move around and find the bacteria.
Speaker 2 Peanut butter, on the other hand, it's oily, not wet, and so the phages have a hard time moving around inside the peanut butter to get at any pathogenic salmonella.
Speaker 2 What this means is that some foods are harder to treat with phages than others.
Speaker 4 But there are still a lot of foods that phages will work on, and there's still a huge potential market for the products that Sandra has developed.
Speaker 2 Which means we haven't even really seen the potential in terms of lives saved yet because phages aren't yet a completely normal part of the food system.
Speaker 4 And then phage researchers think there are still more ways that phages might be able to help fight food poisoning.
Speaker 4 Divya is studying whether people can take phages internally to try to get rid of harmful E. coli in our guts after we get sick or even take them preventatively.
Speaker 4 She's studying whether feeding phages to cattle can help prevent them from shedding so many pathogenic bacteria in their poop. She thinks there's a lot of potential.
Speaker 2 Sandro is also exploring other applications for phages, even phage products for use at home.
Speaker 3 I can see somebody having a spray in their refrigerator that targets one or two or all three or four major pathogens, some in LA coli listeria.
Speaker 3 And if you want to take a remaining lettuce out of your refrigerator, you spray it yourself just an increase precaution and reduce the risk of foodborne illness. So yes, I can see that.
Speaker 3 We are not selling it currently at this point.
Speaker 2 So stay tuned. This phage revolution in our food is just getting started.
Speaker 4 There's even more fascinating research these days about phages and our food system, like whether they can be an early alert about a food that might have become contaminated.
Speaker 4 We've got it all in our special supporters newsletter, gastropod.com/slash support.
Speaker 2 Phages are super hot in general right now. Even Sandro's long-deferred dream of using phages to cure people who are dying from antibiotic-resistant infections, he's finally getting back to that.
Speaker 2 He has a clinical trial running right now. It's one of a handful that are hoping to turn phages into a regular medical treatment for humans in the US finally.
Speaker 4 And meanwhile, some doctors are using phages as the last resort when they have a patient dying of an antibiotic-resistant bacteria like Sandra's boss had.
Speaker 4 It doesn't always work, but sometimes it does.
Speaker 7 A local family got the worst possible news. An infection was taking over a young woman's body and she was close to death.
Speaker 2 Chromophore's Malichen reports willing to try anything, they turn to an obscure treatment from the former Soviet Union.
Speaker 18 From the first phage treatment that we did, the MRSA disappeared. Now, we'd been battling MRSA for almost three years at the point that we started this, and it was gone.
Speaker 4 The MRSA cleared up.
Speaker 2 The Pseudomonas infection went away.
Speaker 4 If phages work, this could be huge. Antibiotic resistance is a growing concern.
Speaker 4 700,000 people die each year because of drug-resistant bacteria, and there are estimates this could rise to 10 million by 2050. Phages could help.
Speaker 2 Phages are starting to get talked about as a miracle cure, and they have saved some lives, but there are still a lot of questions.
Speaker 2 I actually recently wrote an article about people hoping and waiting for phage therapy, but there are a lot of obstacles, including the fact that we don't know what the right dose is, how many phages to give, and how often.
Speaker 4
We can't just start using the phages from Georgia. They're used there for different reasons, for different cases.
This is a new and emerging field.
Speaker 2 And it is exciting, but despite all the hype about human treatment, right now, Sandra says that food safety is really where phages have the biggest impact.
Speaker 3
Food safety is clearly less sexy, so to speak, versus clinical applications. And so there are news stories.
They are more interested in human story.
Speaker 3 They're more interested in finding a person who actually
Speaker 3 was saved by using this bacteriophages. So it was a life-changing event.
Speaker 2 Yep, that was exactly the news story I wrote, too.
Speaker 3 Versus somebody going and treating chickens, even though they potentially save a lot more people than this one single patient.
Speaker 4 Tivya and Sandro are cheerleaders for phages and for food safety, but they're also the first to admit that phages are just part of the solution.
Speaker 4 You have to use them alongside other food safety tools and practices.
Speaker 3 And my hope is that by using bacteriophages, we can either significantly reduce or eliminate use of some of the very harsh interventions, like reduce the use of chemicals, for example, the good for foods, good for us, good for the environment.
Speaker 3
But I don't look at this as a panacea either. I don't think this is a solution to all the problems.
I see this as a part of a multi-hurdle approach that can address some very important issues.
Speaker 2 And frankly, there are some other solutions available to us already that we aren't using.
Speaker 2 In the UK, there was scandal after scandal about salmonella-infected eggs in the 80s and 90s when I was growing up, and so the government decided to vaccinate all British hens, and now you can eat raw eggs with abandon in the UK without fear of getting salmonella.
Speaker 2 But despite the success of the UK program, the US has decided that the cost, which is less than a penny per dozen eggs, means that the vaccine isn't worth it.
Speaker 4
says it's the same with E. coli.
There is a vaccine, but companies aren't willing to pay to give it to their animals. The U.S.
government said zero tolerance, but it's not mandating these vaccines.
Speaker 4
And there are other potential solutions. Animals could live in smaller groups.
Their manure could be processed better.
Speaker 10 So I think, you know, whether it's really exploring grass-fed animals, which we know does lessen the amount of particular kinds of E.
Speaker 10 coli in these animals, whether it's vaccinating chickens, having them in different environments. But all those things have economic implications as well.
Speaker 10 And as a growing population, you have to sort of deal with that.
Speaker 2 In a lot of ways, we have a food safety problem because we have a food system problem.
Speaker 2 This huge centralized system with feedlots and produce shipped all around the country and lots of pre-chopped, pre-washed, pre-processed veggies and meats, it's all set up to make food cheaper and more convenient, but it also encourages the growth and spread of pathogenic bacteria.
Speaker 4 And phages only tackle one problem. It's like a band-aid.
Speaker 4 Phages are great, but they should really be a safeguard for problems that come up, not a way of totally cleaning up the dirty mess of our food system.
Speaker 2 And in fact, that's the real potential problem with phages.
Speaker 2 If they just act as a really great band-aid and clean up all the gross pathogenic bacteria on our food before we eat it, then great, fewer deaths, that's awesome.
Speaker 2 But what's not so great is that we won't have changed anything about the system that created this whole food safety problem in the first place.
Speaker 4 And if people aren't getting sick, they might not care about our food system, which isn't just a foodborne illness problem.
Speaker 4 There are also issues with environmental harm and humane treatment of animals and the safety and well-being of food workers.
Speaker 4 But consumers might forget about those things if they're not getting sick from food.
Speaker 2 I mean, history tells us they will. A critical mass of people really only demand changes to their food system when they're afraid of getting sick.
Speaker 2 It seems to take that personal, selfish motivation to create real change.
Speaker 4 But for now, phages are an amazing tool.
Speaker 4 And frankly, even if we were to make all sorts of changes that would make our food system safer and better for everyone, a girl can dream, we'd still need a tool like phages to mop up some nasty bugs that can show up from time to time.
Speaker 2
Phages are an amazing tool. But also, they're just amazing.
I had never even heard of them before I wrote about them, and now I just want to spout phage facts all day.
Speaker 2 They're behind the scenes, running the show. I used to think microbes were the best.
Speaker 4 Maybe don't drink.
Speaker 2 But phages are their real bosses.
Speaker 4 For instance, these tiny little viruses keep the entire ocean ecosystem in check.
Speaker 3 They trigger about
Speaker 3 septillion infections every second.
Speaker 3 And in that process, they destroy about 40% of bacterial population in the oceans during a single day. So they're really, really very powerful.
Speaker 5 Every time I teach this in my intro microbiology classes, I still get the chills because it's just such a cool way to be a biological entity out there, to be a phage.
Speaker 2 A huge thanks this episode to the Alfred P.
Speaker 2 Sloan Foundation for the public understanding of science, technology, and economics, as well as the Boroughs Welcome Fund for our coverage of biomedical research.
Speaker 4 Thanks this episode to Alexander Sulakuletza, Ben Wolf, Darren Duttweiler, Bill Marlar, and Divia Gironi.
Speaker 4 We have links to everyone's research and books and companies on our website, gastropod.com, as well as to my original article about phages, which is how I met Sandro.
Speaker 2 Also, we have to thank Superstar Gastropod Fellow Sonia Swanson for all her help.
Speaker 4 We'll be back in two weeks with some smoke, some some fire, and some very tasty food. Till then.
Speaker 2 This month on Explain It to Me, we're talking about all things wellness.
Speaker 2 We spend nearly $2 trillion on things that are supposed to make us well: collagen smoothies and cold plunges, Pilates classes, and fitness trackers.
Speaker 2 But what does it actually mean to be well? Why do we want that so badly? And is all this money really making us healthier and happier? That's this month on Explain It To Me, presented by Pureleaf.